Domestic children’s special drugs account for less than 2% of dosage forms, and the specifications are seriously lacking.
Due to the lack of special drugs for children, many children are treated as "miniature adults" when taking drugs. Adult drugs are given to children according to the principle of "children should be reduced at their discretion", and the dosage depends on breaking and guessing. To solve the problem of children’s safe drug use, it is necessary to mobilize all sectors of society to give full play to their respective functional advantages and let children use children’s drugs — —
The recently released White Paper on Children’s Drug Safety Investigation Report in 2016 shows that about 30,000 children in China are deaf every year due to improper drug use. This figure shows that improper drug use by children not only hurts children, but also brings many misfortunes to families and a heavy burden to society.
A few days ago, at the 2017 Children’s Medication Safety Summit Forum jointly sponsored by Children’s Medication Safety Branch of China Medical News Information Association and Beijing Children’s Hospital affiliated to Capital Medical University, experts pointed out that due to the lack of special drugs for children, many children were treated as "scaled-down adults" when taking drugs. According to the principle of "children’s discretion", the use of adult drugs for children was reduced, and the dosage was determined by guessing. "There is a huge safety hazard in doing this, and children should use children’s medicine."
Children’s exclusive drugs are less than 2%
Recently, the reporter visited a number of pharmacies in Beijing and found that many of the common pharmaceutical preparations in the market do not have children’s dosage forms, and children’s special drugs are very scarce. Ms. Liu Hua told reporters that her daughter is three years old this year. Every time she goes to the hospital to see a doctor, she has to do "calculation problems" when she goes home. "The doctor said that children should take less medicine, and they usually take an adult medicine several times. Parents of larger tablets are handcuffed. If the tablets are small, they need to crush them with tools, and then estimate the dose and divide it into several parts for children to take with warm water. " Liu Hua said that compared with adults, children use drugs very little.
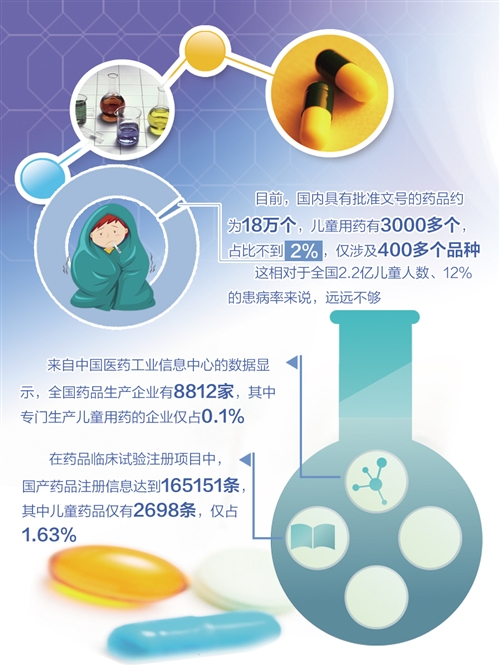
Zhao Zhigang, director of pharmacy department of Tiantan Hospital affiliated to Capital Medical University and chief pharmacist of Beijing Hospital Authority, said that there are about 180,000 drugs with approval numbers in China, and more than 3,000 drugs are used by children, accounting for less than 2%, involving only more than 400 varieties. This is far from enough compared with the number of 220 million children in China and the prevalence rate of 12%. The dosage forms and specifications suitable for children are also very scarce, especially for young children and newborns. According to the survey of Beijing Children’s Hospital affiliated to Capital Medical University, among 231 kinds of commonly used prescription drugs for children, the top three dosage forms are injections, tablets and oral liquid, among which the dosage forms suitable for children, such as powder inhalers, suppositories and syrups, are very few.
Because of children’s vigorous metabolism and poor adjustment ability, their sensitivity to drugs is higher than that of adults, but a large number of drugs are lacking in children’s usage and dosage, and there are few special drugs for children. The incidence of adverse drug reactions among children in China is high. Zhao Zhigang said that the adverse drug reaction rate of children in China was 12.9%, and that of newborns was as high as 24.4%, which was 2 times and 4 times higher than that of adults. The adverse drug reaction report recently released by the State Food and Drug Administration shows that in 2016, the number of children under 14 years old (inclusive) accounted for 10.6% of the total report, of which serious adverse drug reactions accounted for 5.5% of the total report of children.
Drug research and development into a short board of enterprises
Why are there so few special drugs for children? Bian zhenjia, a special researcher in the State Council Counselor’s Office, said: First, the policies are not matched, and the relevant systems of supervision and use are not connected. The marketing approval of children’s drugs is in the food and drug administration department, the policy management is in the health department, the clinical use is in medical institutions, and the reserve management is in the industry and information department. Many parties are responsible for one section, and the relevant systems and standards are not matched, unified and out of line. Second, the clinical trial process is risky, which affects the clinical development of new drugs. This is also a problem that puzzles the children’s medicine production industry in the world. In developed countries, the premise of clinical trials is to sign an informed consent form with volunteers, insure them with accident insurance and necessary economic compensation, and this is the biggest expenditure on research and development. Third, the marketization of children’s medication is not high, the profits of pharmaceutical companies are low, and the enthusiasm for production is not high. At present, the number of children in China and the annual consumption of sick drugs are basically stable, and there is little room for the expansion of the children’s drug market; Children are not miniature versions of adults, their organs are immature, their onset characteristics, process, absorption mechanism and compliance are different, and they also need special formulas, preparations and tastes, which makes the R&D and production process of drugs more complicated and the cost higher, which leads enterprises to be unwilling to engage in the R&D, production and marketing of children-specific drugs.
According to the data from China Pharmaceutical Industry Information Center, there are 8,812 pharmaceutical manufacturers in China, of which only 0.1% are specialized in the production of drugs for children. In the drug clinical trial registration project, the registered information of domestic drugs reached 165,151, of which only 2,698 were children’s drugs, accounting for only 1.63%. Children’s drugs registered in clinical trials only account for 2.35%, which is too little compared with the proportion of more than 20% in developed countries such as the United States.
"Because the pricing principle of children’s medicine is based on the content of active ingredients, the content of active ingredients of children’s medicine is much lower than that of adult medicine, and the corresponding selling price is lower than that of adult medicine, which makes children’s medicine have no obvious advantage in pricing, and enterprises cannot get compensation." Chen Baohua, general manager of Zhejiang Huahai Pharmaceutical Co., Ltd. said that in order to solve the problem of shortage of drugs for children, experts from professional associations can summarize the epidemiological situation and the current situation of children’s drug use in China, determine which pediatric diseases need to be overcome urgently, and formulate a list of children’s drugs to encourage research and development according to domestic and foreign pediatric drug use guidelines and clinical experience, implement priority review and approval, and guide enterprises to research and development.
In addition, relevant experts found in the research that domestic children’s drug instructions generally lack pharmacokinetic data, and even if there are, most of them are foreign children’s data. In some domestic drug instructions, the data of children’s clinical trials basically copy the instructions of imported original drugs. According to experts, there are differences between children and adults, and between children from different countries. China children’s clinical trial data is needed to make the efficacy more accurate, but China’s clinical data is far from enough.
Encourage the development of children’s medicines.
"In order to protect children’s health rights and encourage children’s drug development. In recent years, various departments have formulated various policies on the protection of children’s medication. " Feng Zhang, deputy director of the Department of Pharmacy Administration of the National Health and Family Planning Commission, said that since last year, the Health Planning Commission, the Ministry of Industry and Information Technology and the Food and Drug Administration have published two batches of 71 varieties of children’s drugs to encourage research and development, and made it clear that the drugs in the "list" have been used for many years in Hong Kong, Macao and Taiwan, which are clinically effective and safe to use, but are not listed in the mainland. Explore the pilot import and use of drugs, and allow direct reference to the clinical drug use data of children in Hong Kong, Macao and Taiwan as the basis for declaration when registering and reviewing imported children’s drugs. In addition, through the national "major new drug creation" major science and technology projects, major innovation and development projects for protein biopharmaceuticals and vaccines, we will integrate superior units to collaborate in innovative research and development, and guide and encourage enterprises to give priority to R&D and production; In terms of ensuring production and supply, we will give policy support to the corresponding children’s drug production enterprises and promote product upgrading and technical transformation of production lines.
Wang Tianyou, secretary of the Party Committee of Beijing Children’s Hospital, said that clinical trials of children’s medication are facing difficulties such as difficult evaluation, high evaluation risk, difficulty in recruiting subjects and lack of professionals. During the 13th Five-Year Plan period, Beijing Children’s Hospital will unite a number of units to further strengthen the construction of clinical evaluation support system for children’s demonstration new drugs, and improve the ability of clinical evaluation of children’s drugs through the construction of key technology platforms for children’s drug clinical trials and the construction of key technology platforms for children’s drug big data clinical evaluation. Three clinical specialties were selected as representatives of common diseases, major diseases and rare diseases in children, and a demonstration platform for clinical evaluation of new drugs for children was established.
At the same time, Ceng Yixin, deputy director of the National Health and Family Planning Commission, said that the National Health and Family Planning Commission and other six departments have issued several opinions on the protection of children’s medication and formulated two batches of lists to encourage research and development. Especially in the national major special projects of new drug development, the research and development of children’s drugs and speeding up the examination and approval have been taken as key tasks. "Drugs suitable for children should have appropriate doses according to the weight of different children; The dosage form should be in the form of oral liquid; And it should be suitable for children’s tastes and make them willing to drink. This is ‘ Thirteenth Five-Year Plan ’ Key tasks, the Ministry of Science and Technology, the Ministry of Finance, the Food and Drug Administration, the Health Planning Commission and other joint efforts to speed up the research and development and approval procedures for drugs specially suitable for children in China. It is estimated that the shortage of medicines for children will be effectively solved in a few years. "
"The healthy growth of children is related to the harmony and happiness of each family and the healthy progress and sustainable development of the nation." Feng Zhang said that to break the problem of children’s safe drug use, it is necessary to mobilize all sectors of society to give full play to their respective functional advantages, including government agencies, social organizations, enterprises, hospitals, media, etc., and at the same time, it is also necessary to spread and educate children’s scientific drug use knowledge.